Polio virus is highly stable and persists in the environment for decades, including all three wild-type and vaccine strains. In endemic regions, failure to vaccinate leads inevitably to polio outbreaks. For this reason, vaccination campaigns and vaccine vigilance will be needed worldwide for at least the next three decades*.
The key to control will be worldwide coverage vaccination, including countries with limited technical and financial resources.
 The Need for Innovation
The Need for Innovation
Unfortunately, as the world nears polio extinction, the currently available poliovirus vaccines seem antiquated and inadequate, with a range of problems associated with their use, driving the need for innovation.
 Inactivated Polio Vaccine (IPV)
Inactivated Polio Vaccine (IPV)
 In the USA and most other countries, polio vaccines are manufactured by chemical treatment of virulent polio virus strains, creating an inactivated vaccine, IPV. The IPV vaccine is composed of killed pathogenic strains of the three serotypes and stimulates systemic immunity. Unfortunately, IPV is manufactured by inactivating hazardous, wild-type serotypes with formaldehyde.
In the USA and most other countries, polio vaccines are manufactured by chemical treatment of virulent polio virus strains, creating an inactivated vaccine, IPV. The IPV vaccine is composed of killed pathogenic strains of the three serotypes and stimulates systemic immunity. Unfortunately, IPV is manufactured by inactivating hazardous, wild-type serotypes with formaldehyde.
The use of pathogenic strains in the manufacturing process presents increasingly serious safety and security hazards. In the post-eradication world, the continued use of highly pathogenic strains in vaccine production will raise biosafety and biosecurity concerns.
 Oral Polio Vaccine (OPV)
Oral Polio Vaccine (OPV)
OPV has been the mainstay of eradication efforts. Since its introduction in the 1950s by Dr. Albert Sabin, the use of this live vaccine has resulted in complete global eradication of PV2 and PV3 and near-eradication of PV1. OPV  replicates in the intestine of vaccinees and stimulates robust systemic an d gut immunity. During this replication process, the vaccine strains can lose their attenuated phenotype and revert to pathogenic forms. Recently, the majority of poliomyelitis has been caused by vaccine-derived viruses that have been shed into the environment. In 2019, all paralytic disease caused by serotypes 2 and 3 were due to reversions of attenuated vaccine strains For these reasons, most countries without endemic polio have switched to the non-replicating, injectable IPV.
replicates in the intestine of vaccinees and stimulates robust systemic an d gut immunity. During this replication process, the vaccine strains can lose their attenuated phenotype and revert to pathogenic forms. Recently, the majority of poliomyelitis has been caused by vaccine-derived viruses that have been shed into the environment. In 2019, all paralytic disease caused by serotypes 2 and 3 were due to reversions of attenuated vaccine strains For these reasons, most countries without endemic polio have switched to the non-replicating, injectable IPV.
Both OPV and IPV have remained largely unchanged since their development and deployment in the 1950s. When polio was a global endemic, the risks of natural infection far out-weighed dangers posed by the vaccines. Now that we are close to global eradication, these wonderful vaccines are in need of updates.
BMI’s ultraIPV™ presents one solution to many of these problems.
 WHO Call to Action
WHO Call to Action

Given the importance of maintaining immune protection of all the world’s children while rejecting continued use of current OPV, there exists a global health imperative for the creation and adoption of a safe, low cost, easily manufactured, stable and easy deployed, IPV.
It is anticipated that the use of IPV will increase as countries phase out the use of OPV, however shifting factors in the risk-benefit-cost equation favor the creation of new poliovirus vaccines to be used in the foreseeable future.
In a call to action, the WHO have urged the community to develop safer polio vaccines, ideally based on inactivated Sabin viruses, which are much safer to handle and cannot revert to the wild forms, whose deployment will serve to eradicate polio over the next 30 years. It is anticipated, therefore, that the use of IPV will increase as countries phase out the use of OPV, which can evolve into pathogenic forms.
 Next Generation Solution: ultraIPV™
Next Generation Solution: ultraIPV™
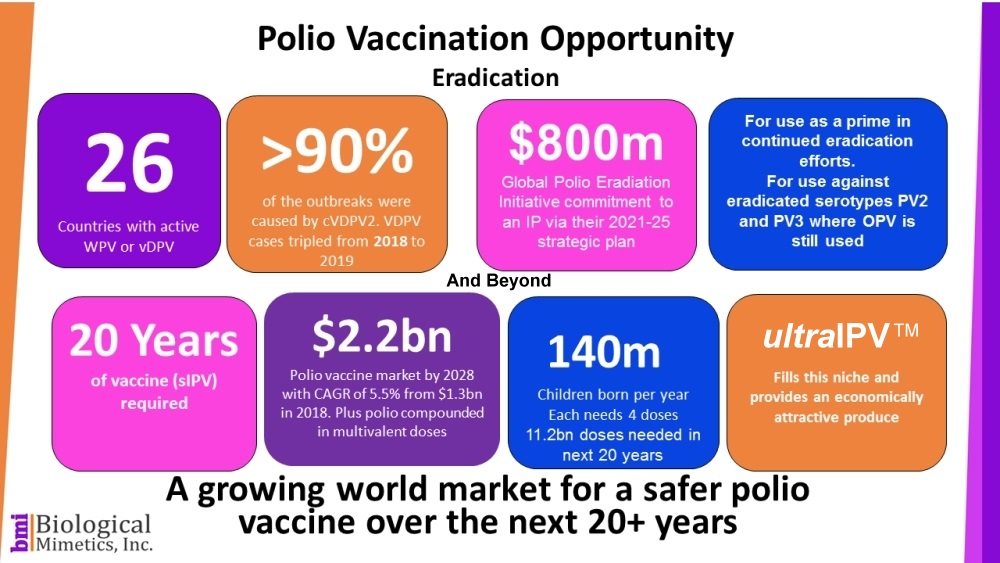
Polio vaccines will be needed worldwide for at least the next 3 decades. Although, Poliovirus Types 2 and 3 have already been eradicated, efforts continue to eradicate Polio 1.
Once eradicated, the WHO advises that countries continue to maintain vaccine vigilance for 20-30 years. After eradication, the biosafety concerns associated with manufacturing IPV from pathogenic strains will become increasingly serious.
![]()
ultraIPV™ is a lower cost IPV and promises to maintain eradication, but with greater global safety and biosecurity compared with existing vaccines. The lower cost is related to the yield of many more vaccine units per milligram of material and a greatly reduced inactivation time (seconds or minutes instead of weeks).
ultraIPV™ has the potential to disrupt and take over a large share of the growing polio vaccine market which is anticipated to record robust CAGR throughout the forecast period, i.e. 2019-2027. The US and EU market is ~$1.5B per year and expected to grow to $4.2B by 2026 with a CAGR of 15.7% (https://marketsgazette24.com).
Refs. * WHO, Gates Foundation

Let's Connect